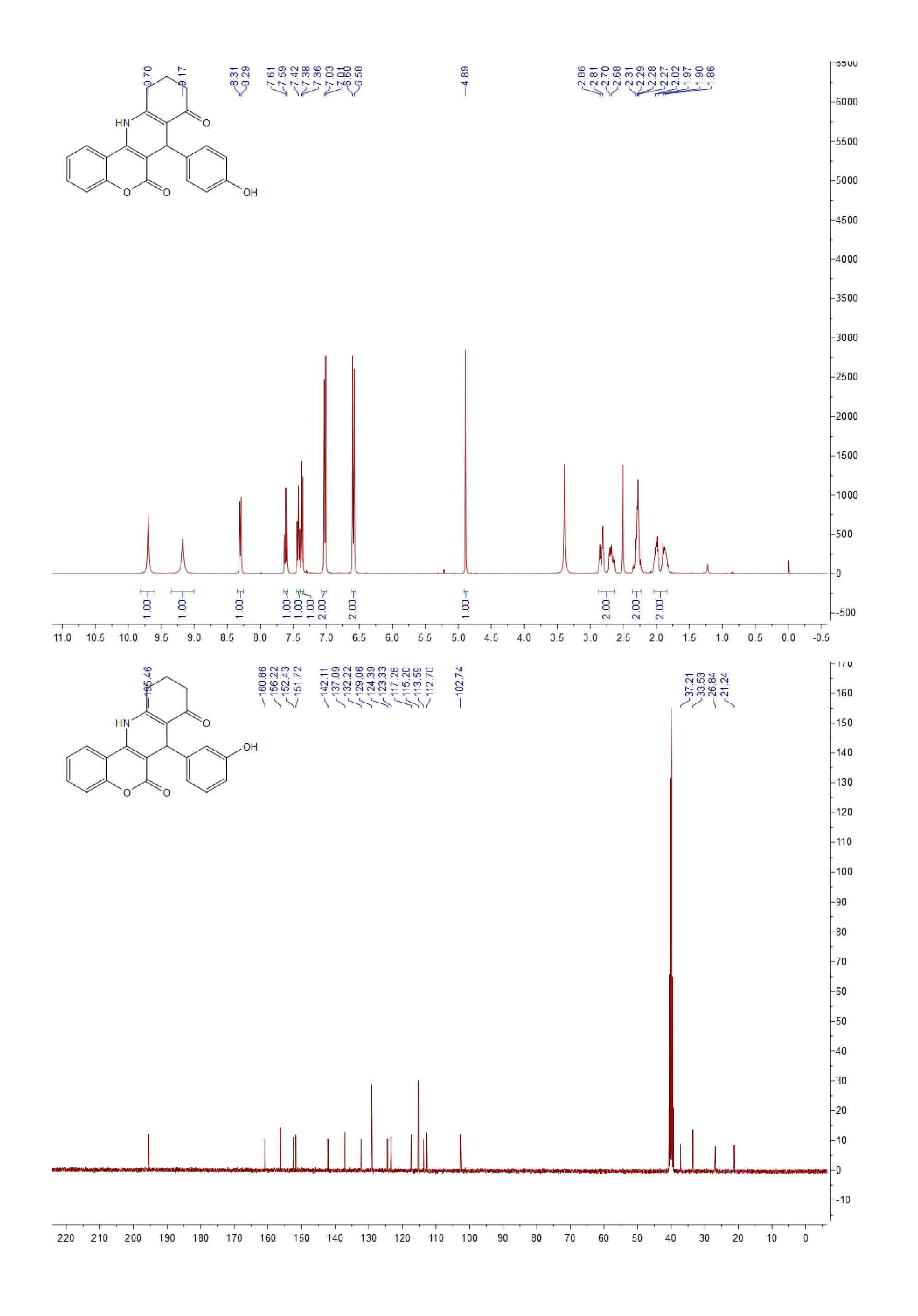
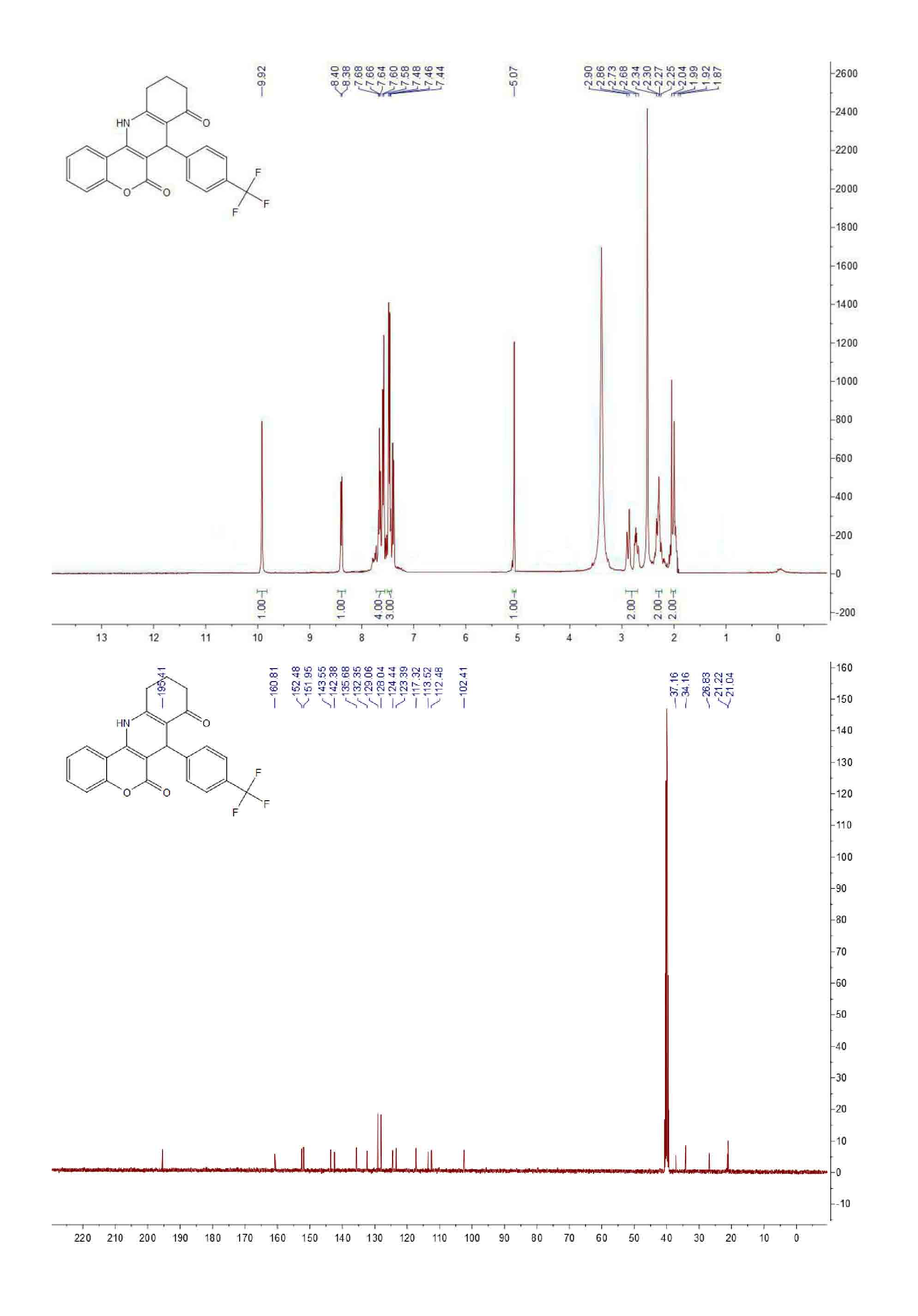
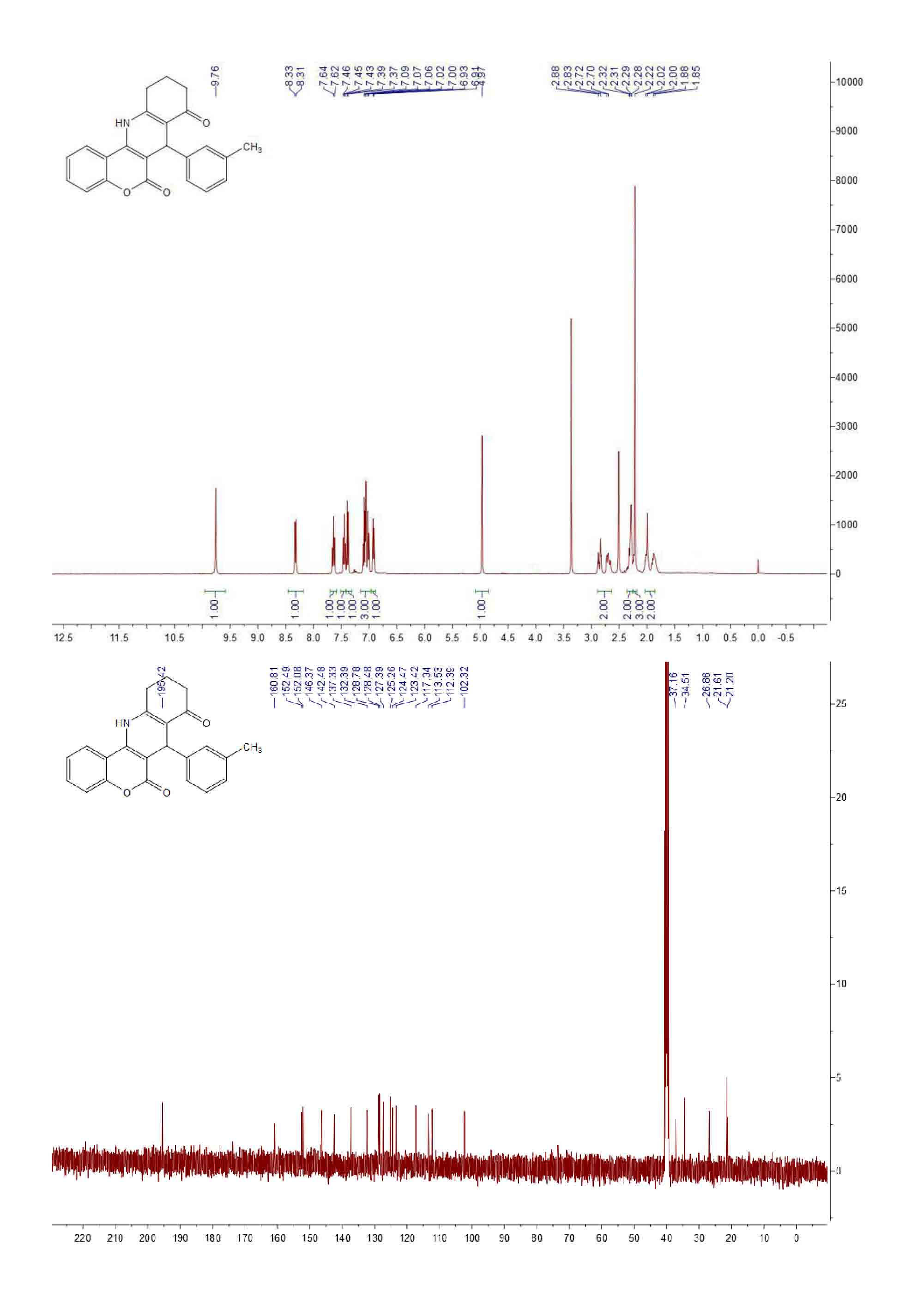
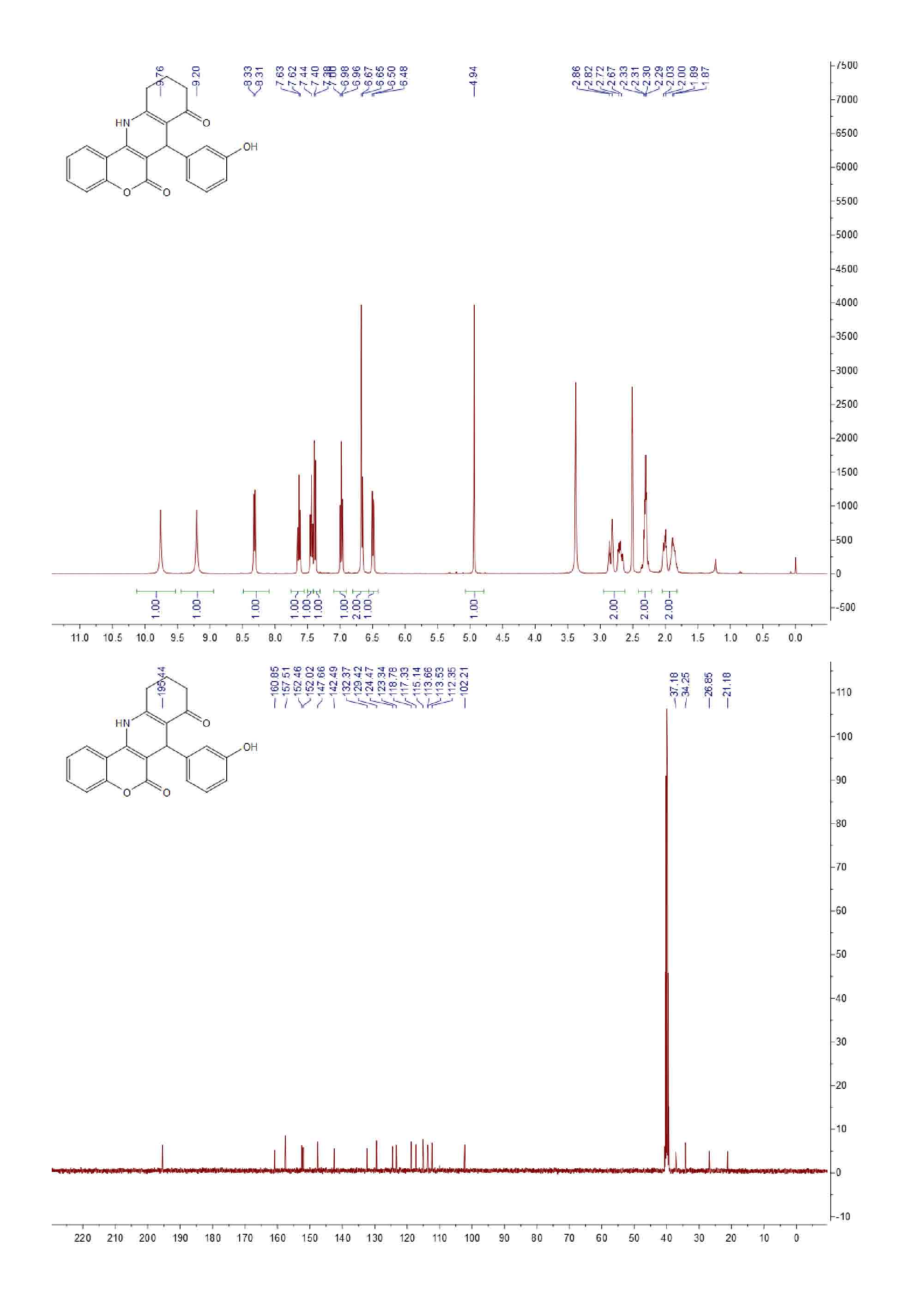
| [1] (a) Anne, A.E.; Konstantina, C.F.; Dimitra, J.H.; Konstantinos, E.L.; Demetrios, N.N. Synthesis and biological evaluation of several 3-(coumarin-4-yl)tetrahydroisoxazole and 3-(coumarin-4-yl)dihydropyrazole derivatives. J. Heterocycl. Chem. 2001, 38, 717–722. (b) Johan, N.; Erik, D.C.; Raghunath, S.; Yung, H.C.; Asish, R.D.; Subhasish, K. Structure-activity relationship of new anti-hepatitis C virus agents: heterobicycle-coumarin conjugates. J. Med. Chem. 2009, 52, 1486–1490.
[2] Angerer, E.V.; Kager, M.; Maucher, A.J. Antitumour activity of coumarin in prostate and mammary cancer models. Cancer Res. Clin. Oncol. 1994, 120, 14–16.
[3] Kirkiacharian, S.; Thuy, D.T.; Sicsic, S.; Bakhchinian, R.; Kurkjian, R.; Tonnaire, T. Chemoselective synthesis of enamino-coumarin derivatives identified as potent antitumor agents. Farmaco. 2002, 57, 703–708.
[4] Sharma, R.C.; Parashar, R.K. Synthesis and microbicidal activity of N-(2-substituted) phenyl ureas and their metal complexes. J. Inorg. Biochem. 1988, 32, 163–169.
[5] Jih, R.H.; Shu, Y.L.; Shwu, C.T.; Erik, D.C.; Pieter, L.; Johan, N. Coumarin-purine ribofuranoside conjugates as new agents against hepatitis C virus. J. Med. Chem. 2011, 54, 2114–2126.
[6] Soroush, S.; Yoki, M.; Kiyoshi, H.; Ronald, G.M.; Sansei, N.; Mohsen, D. Synthesis and antifungal activity of coumarins and angular furano coumarins. Bioorg. Med. Chem. 1999, 7, 1933–1940.
[7] Christos, A.K.; Dimitra, J.H. Synthesis and antiinflammatory activity of coumarin derivatives. J. Med. Chem. 2005, 48, 6400–6408.
[8] Jih, R.H.; Raghunath, S.; Shih, C.H.; Yung, H.C.; Asish, R.D.; Inge, V. Synthesis of new benzimidazole-coumarin conjugates as anti-hepatitis C virus agents. Antiviral. Res. 2008, 77, 157–162.
[9] (a) Mehdi, K.; Masoumeh, A.; Alireza, M.; Amirhossein, S.; Hamid, N.; Seyyede, F.R. Design, synthesis, docking study and biological evaluation of some novel tetrahydrochromeno [3′,4′:5,6]pyrano[2,3-b]quinolin-6(7H)-one derivatives against acetyl- and butyrylcholinesterase. Eur. J. Med. Chem. 2013, 68, 291–300; (b) Bilqees, S.; Mina, S.; Mohammad, M.; Hamid, N.; Farshad, H.M.; Najmeh, E. Synthesis, docking study and neuroprotective effects of some novel pyrano[3,2-c]chromene derivatives bearing morpholine/phenylpiperazine moiety. Bioorg. Med. Chem. 2017, 25, 3980–3988.
[10] (a) Zhi, W.C.; Jian, H.B.; Wei, K.S. Synthesis and antitumor activity of novel coumarin derivatives via a three-component reaction in water. Chin. J. Chem. 2013, 31, 507–514; (b) Ramin, M.; Radineh, M.; Mohammad, R.R.; Omidreza, F.; Azita, J.; Abbas, S. Design, synthesis and evaluation of cytotoxicity of novel chromeno[4,3-b]quinoline derivatives. Arch. Pharm. Chem. Life Sci. 2011, 2, 111–118.
[11] (a) Chao, J.H.; Kan, Z.; Ming, X.; Ting, Y.; Jian, R.G.; Jian, H.J. High quantum yield and pH sensitive fluorescence dyes based on coumarin derivatives: fluorescence characteristics and theoretical study. RSC Adv. 2016, 6, 49221–49227; (b) Radineh, M.; Ghasem, R.B.; Somaye, S. Facile one-pot synthesis of chromeno[4,3-b] quinoline derivatives catalyzed by Cu(II)-Schiff base/SBA-15. Heterocycl. Commun. 2014, 20, 181–184; (c) Naseem, A.; Venkata, B.; Sandeep, S.; Petar, M.M. AN Efficient one-pot three-component synthesis of highly functionslized coumarin fused indenodihydropyridine and chromeno[4,3-b]quinoline derivatives. Heterocycles. 2012, 85, 1629–1653.
[12] Radineh, M.; Sara, S.; Farshid, B. H3PW12O40 as an efficient catalyst for one-pot-tricomponent synthesis of chromeno[4,3-b]quinolines under microwave irradation. Iranian Chem. Commun. 2017, 5, 338–344.
[13] Chao, J.H.; Hao, Z.; Kan, Z.; Ming, X.; Jian, R.G.; Yu, J.L. A novel turn off fluorescent sensor for Fe(III) and pH environment based on coumarin derivatives: the fluorescence characteristics and theoretical study. Tetrahedron. 2016, 72, 8365–8372.
[14] Addison, A.T.; Roberto, M.K.; Kevin, D.B.; Robert, R.L.; James, L.P. Sustained hemodynamic effects of the selective dopamine-1 agonist, fenoldopam, during 48-hour infusions in hypertensive patients: a dose-tolerability study. J. Clin. Pharmacol .1999, 39, 471–479.
[15] (a) Robert, M. Quinoline-based HIV integrase inhibitors. Curr. Pharm. Des. 2013, 19, 1835–1849; (b) Suresh, K.; Sandhya, B.; Himanshu, G. Biological activities of quinoline derivatives. Mini-Rev. Med. Chem. 2009, 9, 1648–1654; (c) Alexander, D.; Wei, W.; Kan, W. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135; (d) Samahe, S.; Majid, M.H. Recent application of isocyanides in synthesis of heterocycles. Tetrahedron. 2011, 67, 2707–2752; (e) Hua, J.Z.; Wen, J.C.; Xin, H.; Lai, S.W. On the electronic structure and conflicting d-orbital aromaticity in the Re3O3-cluster. RSC Adv. 2012, 2, 9763–9777; (f) Nicolas, I.; Rodolfo, L. Heterocycles as key substrates in multicomponent reactions: the fast lane towards molecular complexity. Chem. Eur. J. 2008, 14, 8444–8454; (g) Barry, B.T.; Dennis, G.H. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 2009, 109, 4439–4486.
[16] (a) Gary, H.P. Multicomponent one-pot annulations forming 3 to 6 bonds. Chem. Rev. 1986, 86, 831–844; (b) Robert, W.A.; Andrew, P.C.; Paul, A.T.; David, B.; Thomas, A.K. Multiple-component condensation strategies for combinatorial library synthesis. Acc. Chem. Res. 1996, 29, 123–131; (c) Hugues, B.; Chris, H.; Gilles, O.; Philippe, S. Maximizing synthetic efficiency: multi-component transformations lead the way. Chem. Eur. J. 2000, 6, 3321–3329; (d) Jie, P.Z. Recent developments in the isonitrile-based multicomponent synthesis of heterocycles. Eur. J. Org. Chem. 2003, 7, 1133–1144; (e) Alexander, D. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89.
[17] Dong, C.; Xie, L.L.; Mou, H.H.; Zhong, Y.; Su, W. Facile synthesis of 1,3,4-benzotriazepines and 1-arylamide-1H-indazoles via palladium-catalyzed cyclization of aryl isocyanates and aryl hydrazones under microwave irradiation. Org. Biomol. Chem. 2010, 8, 4827–4830.
[18] Zheng, J.; He, M.; Xie, B.H.; Yang, L.; Hu, Z.; Zhou, H.; Dong, C. Enantioselective synthesis of novel pyrano[3,2-c]chromene derivatives as AChE inhibitors via an organocatalytic domino reaction. Org. Biomol. Chem. 2018, 16, 472–479.
[19] (a) Hong, J.W.; Li, P.M.; Zhan, H.Z. Cerium ammonium nitrate-catalyzed multicomponent reaction for efficient synthesis of functionalized tetrahydropyridines. ACS Comb. Sci. 2011, 13, 181–185; (b) Chitreddy, S.V.; Shanmugam, S. Solvent free-synthesis of highly functionalized 4H-chromene-3-carboxamide derivatives using cerium ammonium nitrate and their antioxidant, antibacterial and solvatochromism studies. J. Mol. Liquids. 2017, 243, 494–502; (c) Cherkupally, S.R.; Mekala, R. Cerium(IV) ammonium nitrate catalysed facile and efficient synthesis of polyhydroquinoline derivatives through Hantzsch multicomponent condensation. Chin. Chem. Lett. 2008, 19, 775–779.
[20] George, L.E.; Diane, C.; Valentino, A.; Robert, M.F. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90.
[21] Oleg, T.; Arthur, J.O. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–460. |
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)